 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
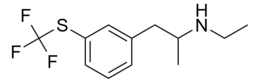
| Formula | C12H16F3NS |
| Molar mass | 263.32 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Tiflorex (TFX), formerly known as flutiorex, is a stimulant[citation needed] amphetamine that was under development as an appetite suppressant in the 1970s,[1][2] but appears to have been abandoned. It is structurally related to fenfluramine and 4-MTA.
Tiflorex went to phase II clinical trials. The extended release formulation "TFX-SR" produced significant suppression of appetite. It also caused slightly more sleep disturbances and headaches than placebo, as well as mydriasis and a self-reported decrease in arousal. It had little effect on heart rate.[2]
Tifluorex is claimed to be a more potent anorectic than fenfluramine, with twice its potency in humans[2] and 4 times its potency in rats.[3]
- ^ Giudicelli JF, Richer C, Berdeaux A (February 1976). "Preliminary assessment of flutiorex, a new anorectic drug, in man". British Journal of Clinical Pharmacology. 3 (1): 113–21. doi:10.1111/j.1365-2125.1976.tb00578.x. PMC 1428817. PMID 788737.
- ^ a b c Silverstone T, Fincham J, Plumley J (April 1979). "An evaluation of the anorectic activity in man of a sustained release formulation of tiflorex". British Journal of Clinical Pharmacology. 7 (4): 353–6. doi:10.1111/j.1365-2125.1979.tb00945.x. PMC 1429648. PMID 444355.
- ^ Stuart S (2013-09-11). Abstracts: Sixth International Congress of Pharmacology. Elsevier. ISBN 9781483152530.