 | |
| Names | |
|---|---|
| Other names
titanium tetranitrate, tetranitratotitanium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.222.601 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ti(NO3)4 | |
| Molar mass | 295.8866 g/mol |
| Appearance | white volatile solid |
| Density | 2.192[3] |
| Melting point | 58[4] °C (136 °F; 331 K) |
| Boiling point | decompose |
| Reacts[5] | |
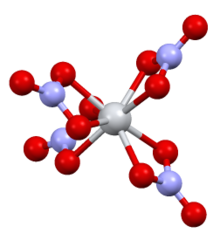
| Structure[6] | |
| monoclinic | |
| P21/C | |
a = 7.80, b = 13.57, c = 10.34 Å α = 90°, β = 125·0°, γ = 90°
| |
Lattice volume (V)
|
896.52 Å3 |
Formula units (Z)
|
4 |
| 8 | |
| flattened tetrahedral | |
| Related compounds | |
Related compounds
|
hafnium nitrate, zirconium nitrate, titanium phosphate, titanium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
- ^ Garner, C. D.; Wallwork, S. C. (1966). "The crystal structures of anhydrous nitrates and their complexes. Part III. Titanium(IV) nitrate". J. Chem. Soc. A: 1496–1500. doi:10.1039/J19660001496.
- ^ "ICSD 26639 : ICSD Structure : N4 O12 Ti". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. Retrieved 2021-05-08.
- ^ "Titanium(iv) nitrate (Ti(NO3)4)". Retrieved 27 September 2014.
- ^ Chemistry of the Elements (Second Edition). N. N. Greenwood and A. Earnshaw. P966. 21.3.4 Compounds with oxoanions
- ^ Nathaniel Howell Furman; R. J. Mundy; G. H. Morrison (1955). The Distribution of Uranyl Nitrate from Aqueous Solutions to Diethyl Ether. the University of Michigan: U.S. Atomic Energy Commission. Technical Information Service. p. 51.
- ^ Garner, C. David; Ian H. Hillier; Martyn F. Guest (1975). "Ab initio self-consistent field molecular-orbital calculation of the ground state of tetranitratotitanium(IV); comments on the reactivity of anhydrous metal nitrates". Journal of the Chemical Society, Dalton Transactions (19): 1934. doi:10.1039/DT9750001934. ISSN 0300-9246.