| |
| |
| Names | |
|---|---|
| IUPAC name
Titanium(IV) bromide
| |
| Other names
Titanium tetrabromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.029.259 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TiBr4 | |
| Molar mass | 367.483 g/mol |
| Appearance | brown crystals hygroscopic |
| Density | 3.25 g/cm3 |
| Melting point | 39 °C (102 °F; 312 K) |
| Boiling point | 230 °C (446 °F; 503 K) |
| hydrolyses | |
| Solubility in other solvents | chlorocarbons, benzene |
| Structure | |
| cubic, Pa3, Z = 8 | |
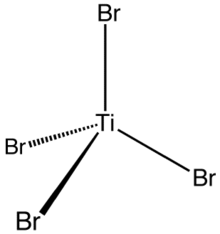
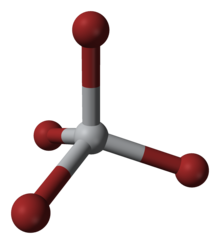
| Tetrahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
corrosive |
| GHS labelling:[1] | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P363, P405 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Titanium(IV) chloride Titanium(IV) fluoride Titanium(IV) iodide |
Related compounds
|
Titanium(III) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Titanium tetrabromide is the chemical compound with the formula TiBr4. It is the most volatile transition metal bromide. The properties of TiBr4 are an average of TiCl4 and TiI4. Some key properties of these four-coordinated Ti(IV) species are their high Lewis acidity and their high solubility in nonpolar organic solvents. TiBr4 is diamagnetic, reflecting the d0 configuration of the metal centre.[2]
- ^ "Titanium tetrabromide". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
