 | |
| Clinical data | |
|---|---|
| Trade names | Xeljanz, Jaquinus, Tofacinix, Others |
| Other names | CP-690550 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613025 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Janus kinase (JAK) inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 74% |
| Protein binding | 40% |
| Metabolism | Liver (via CYP3A4 and CYP2C19) |
| Elimination half-life | 3 hours |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.928 |
| Chemical and physical data | |
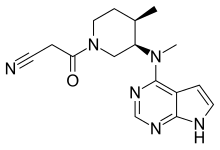
| Formula | C16H20N6O |
| Molar mass | 312.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis.[8][9][10] It is a janus kinase (JAK) inhibitor,[8][9] discovered and developed by the National Institutes of Health and Pfizer.
Common side effects include diarrhea, headache, and high blood pressure.[10] Serious side effects may include infections, cancer, and pulmonary embolism.[10][11] In 2019, the safety committee of the European Medicines Agency began a review of tofacitinib and recommended that doctors temporarily not prescribe the 10 mg twice-daily dose to people at high risk for pulmonary embolism.[12] The U.S. Food and Drug Administration (FDA) also released warnings about the risk of blood clots.[13][14][15] An important side effect of Jakinibs is serious bacterial, mycobacterial, fungal and viral infections. In the phase III trials of tofacitinib among opportunistic infections, pulmonary tuberculosis (TB) was reported in 3 cases all of which were initially negative upon screening for TB.[16]
It was approved for medical use in the United States in November 2012.[17] The extended release version was approved in February 2016.[18] It is available as a generic medication.[19]
- ^ "Tofacitinib Use During Pregnancy". Drugs.com. 15 April 2020. Retrieved 23 October 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Xeljanz/Xeljanz XR (Pfizer Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 16 February 2023. Retrieved 10 April 2023.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2015". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Product monograph brand safety updates". Health Canada. 6 June 2024. Retrieved 8 June 2024.
- ^ "10 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 13 October 2020. Retrieved 3 November 2020.
- ^ "Xeljanz 11 mg prolonged release tablets - Summary of Product Characteristics (SmPC)". (emc). Retrieved 3 November 2020.
- ^ a b c Cite error: The named reference
Xeljanz FDA labelwas invoked but never defined (see the help page). - ^ a b c "Xeljanz EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 3 November 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c "Tofacitinib Citrate". The American Society of Health-System Pharmacists. Retrieved 1 June 2018.
- ^ "Safety Alerts for Human Medical Products - Xeljanz, Xeljanz XR (tofacitinib): Safety Communication - Safety Trial Finds Increased Risk of Blood Clots in the Lungs and Death with Higher Dose in Rheumatoid Arthritis Patients". U.S. Food and Drug Administration (FDA). Retrieved 2 March 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 13-16 May 2019, May 17, 2019". European Medicines Agency. 17 May 2019. Retrieved 17 May 2019.
- ^ "Xeljanz, Xeljanz XR (tofacitinib): Drug Safety Communication - Due to an Increased Risk of Blood Clots and Death with Higher Dose". U.S. Food and Drug Administration (FDA). 26 July 2019. Archived from the original on 15 December 2019. Retrieved 10 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). U.S. Food and Drug Administration (FDA) (Podcast). 5 August 2019. Retrieved 15 December 2019.
- ^ "FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR)". U.S. Food and Drug Administration. 15 December 2019. Archived from the original on 15 December 2019. Retrieved 15 December 2019.
- ^ O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A (April 2013). "Janus kinase inhibitors in autoimmune diseases". Annals of the Rheumatic Diseases. 72 (Suppl 2): ii111–ii115. doi:10.1136/annrheumdis-2012-202576. PMC 3616338. PMID 23532440.
- ^ "Drug Approval Package: Xeljanz (tofacitinib) Tablets NDA #203214". U.S. Food and Drug Administration (FDA). 28 December 2012. Retrieved 30 June 2023.
- ^ "Drug Approval Package: Xeljanz (tofacitinib) Extended Release (XR) Tablets NDA #208246". U.S. Food and Drug Administration (FDA). 26 June 2017. Retrieved 30 June 2023.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.