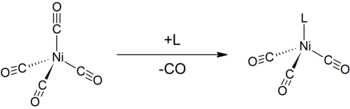The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode (ν(CO)) of a (pseudo)-C3v symmetric complex, [LNi(CO)3] by infrared spectroscopy, where L is the ligand of interest. [LNi(CO)3] was chosen as the model compound because such complexes are readily prepared from tetracarbonylnickel(0).[1][2] The shift in ν(CO) is used to infer the electronic properties of a ligand, which can aid in understanding its behavior in other complexes. The analysis was introduced by Chadwick A. Tolman.
The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by π backbonding: the metal forms a π bond with the carbonyl ligand by donating electrons through its d orbitals into the empty π* anti-bonding orbitals on CO. This interaction strengthens the metal-carbon bond but also weakens the carbon-oxygen bond, resulting in a lower vibrational frequency. If other ligands increase the density of π electrons on the metal, the C-O bond is weakened and ν(CO) decreases further; conversely, if other ligands compete with CO for π backbonding, ν(CO) increases.

| L | ν(CO) cm−1 |
|---|---|
| P(t-Bu)3 | 2056.1 |
| P(NMe2)3 | 2061.9 |
| PMe3 | 2064.1 |
| P(C6H4OMe)3 | 2066 |
| PPh3 | 2068.9 |
| P(C6H4F)3 | 2071.3 |
| P(OEt)3 | 2076.3 |
| PCl3 | 2097.0 |
| PF3 | 2110.8 |
- ^ Robert H. Crabtree (2005). "Carbonyls, Phosphine Complexes, and Ligand Substitution Reactions". The Organometallic Chemistry of the Transition Metals. pp. 87–124. doi:10.1002/0471718769.ch4. ISBN 9780471718765.
- ^ a b Tolman, C. A. (1977). "Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis". Chem. Rev. 77 (3): 313–348. doi:10.1021/cr60307a002.