| Transferrin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Transferrin | ||||||||
| Pfam | PF00405 | ||||||||
| InterPro | IPR001156 | ||||||||
| PROSITE | PDOC00182 | ||||||||
| SCOP2 | 1lcf / SCOPe / SUPFAM | ||||||||
| |||||||||
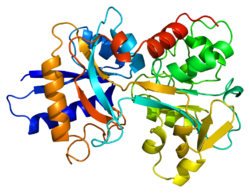
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma.[5] They are produced in the liver and contain binding sites for two Fe3+ ions.[6] Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.[7][8]
Transferrin glycoproteins bind iron tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of total body iron, it forms the most vital iron pool with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 kDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high (association constant is 1020 M−1 at pH 7.4)[9] but decreases progressively with decreasing pH below neutrality. Transferrins are not limited to only binding to iron but also to different metal ions.[10] These glycoproteins are located in various bodily fluids of vertebrates.[11][12] Some invertebrates have proteins that act like transferrin found in the hemolymph.[11][13]
When not bound to iron, transferrin is known as "apotransferrin" (see also apoprotein).
- ^ a b c GRCh38: Ensembl release 89: ENSG00000091513 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000032554 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Crichton RR, Charloteaux-Wauters M (May 1987). "Iron transport and storage". European Journal of Biochemistry. 164 (3): 485–506. doi:10.1111/j.1432-1033.1987.tb11155.x. PMID 3032619.
- ^ Hall DR, Hadden JM, Leonard GA, Bailey S, Neu M, Winn M, Lindley PF (January 2002). "The crystal and molecular structures of diferric porcine and rabbit serum transferrins at resolutions of 2.15 and 2.60 A, respectively". Acta Crystallographica. Section D, Biological Crystallography. 58 (Pt 1): 70–80. Bibcode:2002AcCrD..58...70H. doi:10.1107/s0907444901017309. PMID 11752780.
- ^ Yang F, Lum JB, McGill JR, Moore CM, Naylor SL, van Bragt PH, et al. (May 1984). "Human transferrin: cDNA characterization and chromosomal localization". Proceedings of the National Academy of Sciences of the United States of America. 81 (9): 2752–6. Bibcode:1984PNAS...81.2752Y. doi:10.1073/pnas.81.9.2752. PMC 345148. PMID 6585826.
- ^ Kawabata H (March 2019). "Transferrin and transferrin receptors update". Free Radical Biology & Medicine. 133: 46–54. doi:10.1016/j.freeradbiomed.2018.06.037. PMID 29969719. S2CID 49674402.
- ^ Aisen P, Leibman A, Zweier J (March 1978). "Stoichiometric and site characteristics of the binding of iron to human transferrin". The Journal of Biological Chemistry. 253 (6): 1930–7. doi:10.1016/S0021-9258(19)62337-9. PMID 204636.
- ^ Nicotra S, Sorio D, Filippi G, De Gioia L, Paterlini V, De Palo EF, et al. (November 2017). "Terbium chelation, a specific fluorescent tagging of human transferrin. Optimization of conditions in view of its application to the HPLC analysis of carbohydrate-deficient transferrin (CDT)". Analytical and Bioanalytical Chemistry. 409 (28): 6605–6612. doi:10.1007/s00216-017-0616-z. PMID 28971232. S2CID 13929228.
- ^ a b MacGillivray RT, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, et al. (June 1998). "Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release". Biochemistry. 37 (22): 7919–28. doi:10.1021/bi980355j. PMID 9609685.
- ^ Dewan JC, Mikami B, Hirose M, Sacchettini JC (November 1993). "Structural evidence for a pH-sensitive dilysine trigger in the hen ovotransferrin N-lobe: implications for transferrin iron release". Biochemistry. 32 (45): 11963–8. doi:10.1021/bi00096a004. PMID 8218271.
- ^ Baker EN, Lindley PF (August 1992). "New perspectives on the structure and function of transferrins". Journal of Inorganic Biochemistry. 47 (3–4): 147–60. doi:10.1016/0162-0134(92)84061-q. PMID 1431877.