 | |
| Clinical data | |
|---|---|
| Trade names | Finajet, Finaplix, others |
| Other names | RU-1697; Trenbolone 17β-acetate; 19-Nor-δ9,11-testosterone 17β-acetate; Estra-4,9,11-trien-17β-ol-3-one 17β-acetate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Pharmacokinetic data | |
| Elimination half-life | Intramuscular: 3 days[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.030.380 |
| Chemical and physical data | |
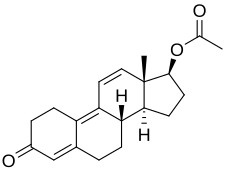
| Formula | C20H24O3 |
| Molar mass | 312.409 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trenbolone acetate, sold under brand names such as Finajet and Finaplix among others, is an androgen and anabolic steroid (AAS) medication used in veterinary medicine, specifically to increase the profitability of livestock by promoting muscle growth in cattle.[2][3][4][5] It is given by injection into muscle.[5][2]
Side effects of trenbolone acetate include symptoms of masculinization like acne, increased body hair growth, scalp hair loss, voice changes, and increased sexual desire.[5] The drug is a synthetic androgen and anabolic steroid[6] and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[5][2][7] It has strong anabolic effects and highly androgenic effects, as well as potent progestogenic effects, and weak glucocorticoid effects.[5][2][7][8][9] Trenbolone acetate is an androgen ester and a short-lasting prodrug of trenbolone in the body.
Trenbolone acetate was discovered in 1963 and was introduced for veterinary use in the early 1970s.[5][10][11] In addition to its veterinary use, trenbolone acetate is used to improve physique and performance, for which purpose it is purchased from black market suppliers.[5] The drug is a controlled substance in many countries and so non-veterinary use is generally illicit.[5]
- ^ Ruiz P, Strain EC (2011). Lowinson and Ruiz's Substance Abuse: A Comprehensive Textbook. Lippincott Williams & Wilkins. pp. 358–. ISBN 978-1-60547-277-5.
- ^ a b c d Yarrow JF, McCoy SC, Borst SE (June 2010). "Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity". Steroids. 75 (6): 377–389. doi:10.1016/j.steroids.2010.01.019. PMID 20138077. S2CID 205253265.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 279–. ISBN 978-94-011-4439-1.
- ^ "Trenbolone". Archived from the original on 2020-07-07. Retrieved 2017-11-11.
- ^ a b c d e f g h Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 491–499. ISBN 978-0-9828280-1-4.
- ^ Zarkawi M, Galbraith H, Hutchinson JS (April 1991). "The action of trenbolone acetate, a synthetic anabolic steroid, on ovarian function in the guinea pig". Laboratory Animals. 25 (2): 117–121. doi:10.1258/002367791781082586. PMID 1857092. S2CID 31765638.
- ^ a b Kicman AT (June 2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ Cite error: The named reference
MeyerRapp1985was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid7382482was invoked but never defined (see the help page). - ^ Robinson JA, Ma Q, Staveley JP, Smolenski WJ, Ericson J (March 2017). "Degradation and transformation of 17α-trenbolone in aerobic water-sediment systems". Environmental Toxicology and Chemistry. 36 (3): 630–635. doi:10.1002/etc.3381. PMID 26800846. S2CID 4706788.
- ^ Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, et al. (April 2006). "Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot". Environmental Health Perspectives. 114 (S-1): 65–68. doi:10.1289/ehp.8055. PMC 1874171. PMID 16818248.