 | |
| Clinical data | |
|---|---|
| Other names | MENT; MENTR; RU-27333; 7α-Methylnandrolone; 7α-Methyl-19-nortestosterone; 7α-Methylestr-4-en-17β-ol-3-one |
| Routes of administration | Subcutaneous implant, intramuscular injection (as trestolone acetate) |
| Drug class | Androgen; Anabolic steroid; Progestogen; Antigonadotropin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.184.887 |
| Chemical and physical data | |
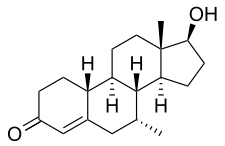
| Formula | C19H28O2 |
| Molar mass | 288.431 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use.[1][2][3][4][5] It is given as an implant that is placed into fat.[3] As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.[1][5]
Side effects Trestolone is an AAS, and hence is an agonist of the androgen receptor, the biological target of androgens like testosterone.[3][6] It is also a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[3][6] Due to its androgenic and progestogenic activity, trestolone has antigonadotropic effects.[3][6] These effects result in reversible suppression of sperm production and are responsible for the contraceptive effects of trestolone in men.[3]
Trestolone was first described in 1963.[7] Subsequently, it was not studied again until 1990.[8] Development of trestolone for potential clinical use started by 1993 and continued thereafter.[4][9] No additional development appears to have been conducted since 2013.[3] The medication was developed by the Population Council, a non-profit, non-governmental organization dedicated to reproductive health.[3][10]
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 888–. ISBN 978-1-4757-2085-3.
- ^ "7-alpha-methyl-19-nortestosterone - AdisInsight".
- ^ a b c d e f g h Nieschlag E, Kumar N, Sitruk-Ware R (2013). "7α-methyl-19-nortestosterone (MENTR): the population council's contribution to research on male contraception and treatment of hypogonadism". Contraception. 87 (3): 288–95. doi:10.1016/j.contraception.2012.08.036. PMID 23063338.
- ^ a b Cite error: The named reference
pmid8489761was invoked but never defined (see the help page). - ^ a b Corona G, Rastrelli G, Vignozzi L, Maggi M (2012). "Emerging medication for the treatment of male hypogonadism". Expert Opin Emerg Drugs. 17 (2): 239–59. doi:10.1517/14728214.2012.683411. PMID 22612692. S2CID 22068249.
- ^ a b c Cite error: The named reference
pmid22065861was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid13931986was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid2363352was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid8146434was invoked but never defined (see the help page). - ^ MENT – project information from the Population Council