| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trifluoromethanesulfonic acid
| |||
| Other names
Triflic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.014.625 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
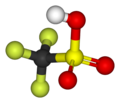
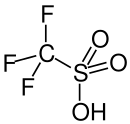
| CF3SO3H | |||
| Molar mass | 150.07121 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.696 g/mL | ||
| Melting point | −40 °C (−40 °F; 233 K) | ||
| Boiling point | 162 °C (324 °F; 435 K) | ||
| 1600 g/L | |||
| Vapor pressure | 3.2 | ||
| Acidity (pKa) | −14.7±2.0[1] | ||
| Conjugate base | Triflate anion | ||
| Viscosity | 1.864–1.881 mm2/s at 20 °C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Causes severe acid burns | ||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H290, H302, H314, H335, H402 | |||
| P234, P261, P264, P270, P271, P273, P280, P301+P312+P330, P301+P330+P331, P303+P361+P353, P304+P340+P310, P305+P351+P338+P310, P363, P390, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification.[2][3] It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.
- ^ Trummal, A.; Lipping, L.; Kaljurand, I.; Koppel, I. A.; Leito, I. (2016). "Acidity of Strong Acids in Water and Dimethyl Sulfoxide". Journal of Physical Chemistry A. 120 (20): 3663–3669. Bibcode:2016JPCA..120.3663T. doi:10.1021/acs.jpca.6b02253. PMID 27115918. S2CID 29697201.
- ^ Howells, R. D.; McCown, J. D. (1977). "Trifluoromethanesulfonic Acid and Derivatives". Chemical Reviews. 77 (1): 69–92. doi:10.1021/cr60305a005.
- ^ Subramanian, L. R.; Martinez, A. G.; Hanack, M.; Prakash, G. K. S.; Hu, J. (2006). "Trifluoromethanesulfonic Acid". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rt246.pub2. ISBN 0-471-93623-5.