 | |
| Clinical data | |
|---|---|
| Trade names | Viroptic; Lonsurf (+tipiracil) |
| Other names | α,α,α-trifluorothymidine; 5-trifluromethyl-2′-deoxyuridine; FTD5-trifluoro-2′-deoxythymidine; TFT; CF3dUrd; FTD; F3TDR; F3Thd |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Eye drops; tablets (+tipiracil) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Negligible (eye drops); ≥57% (oral) |
| Protein binding | >96% |
| Metabolism | Thymidine phosphorylase |
| Elimination half-life | 12 minutes (eye drops); 1.4–2.1 hrs (combination with tipiracil) |
| Excretion | Mostly via urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.657 |
| Chemical and physical data | |
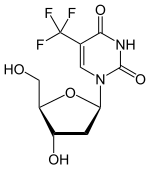
| Formula | C10H11F3N2O5 |
| Molar mass | 296.202 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Trifluridine (also called trifluorothymidine; abbreviation TFT or FTD[1]) is an anti-herpesvirus antiviral drug, used primarily as prescription eyedrops. It was sold under the trade name Viroptic by Glaxo Wellcome, now merged into GlaxoSmithKline. The brand is now wholly owned by King Pharmaceuticals.
Trifluridine was approved for medical use in 1980.[2] It is also a component of the anti-cancer drug trifluridine/tipiracil, which is taken by mouth.
- ^ Patel AK, Abhyankar R, Brais LK, Duh MS, Barghout VE, Huynh L, et al. (December 2021). "Trifluridine/Tipiracil and Regorafenib in Patients with Metastatic Colorectal Cancer: A Retrospective Study at a Tertiary Oncology Center". The Oncologist. 26 (12): e2161–e2169. doi:10.1002/onco.13942. PMC 8649060. PMID 34406678.
- ^ Kimberlin DW (2012). "Antiviral Agents". In Long SS, Pickering LK, Prober CG (eds.). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 1502. ISBN 978-1437727029.