 | |
 | |
 | |
| Names | |
|---|---|
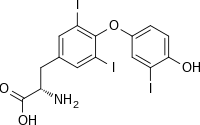
| IUPAC name
(2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid
| |
| Other names
triiodothyronine
T3 3,3′,5-triiodo-L-thyronine | |
| Identifiers | |
3D model (JSmol)
|
|
| 2710227 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.027.272 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H12I3NO4 | |
| Molar mass | 650.977 g·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triiodothyronine, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate.[1]
Production of T3 and its prohormone thyroxine (T4) is activated by thyroid-stimulating hormone (TSH), which is released from the anterior pituitary gland. This pathway is part of a closed-loop feedback process: Elevated concentrations of T3, and T4 in the blood plasma inhibit the production of TSH in the anterior pituitary gland. As concentrations of these hormones decrease, the anterior pituitary gland increases production of TSH, and by these processes, a feedback control system stabilizes the level of thyroid hormones in the bloodstream.
At the cellular level, T3 is the body's more active and potent thyroid hormone.[2] T3 helps deliver oxygen and energy to all of the body's cells, its effects on target tissues being roughly four times more potent than those of T4.[2] Of the thyroid hormone that is produced, just about 20% is T3, whereas 80% is produced as T4. Roughly 85% of the circulating T3 is later formed in the liver and anterior pituitary by removal of the iodine atom from the carbon atom number five of the outer ring of T4. In any case, the concentration of T3 in the human blood plasma is about one-fortieth that of T4. The half-life of T3 is about 2.5 days.[3] The half-life of T4 is about 6.5 days.[4] T3 levels start to rise 45 minutes after administration and peak at about 2.5 hours. Although manufacturer of Cytomel states half-life to be 2.5 days the half-life variability is great and can vary depending on the thyroid status of the patient. Newer studies have found the pharmakokinetics of T3 to be complex and the half-life to vary between 10 – 22 hours.[5]
- ^ Bowen R (2010-07-24). "Physiologic Effects of Thyroid Hormones". Colorado State University. Retrieved 2013-09-29.
- ^ a b "How Your Thyroid Works – "A delicate Feedback Mechanism"". endocrineweb. 2012-01-30. Retrieved 2013-09-29.
- ^ "Cytomel (Liothyronine Sodium) Drug Information". RxList. 2011-01-03. Retrieved 2013-09-29.
- ^ Irizarry L (23 April 2014). "Thyroid Hormone Toxicity". Medscape. WedMD LLC. Retrieved 2 May 2014.
- ^ Jonklaas J, Burman KD, Wang H, Latham KR (February 2015). "Single Dose T3 Administration: Kinetics and Effects on Biochemical and Physiologic Parameters". Therapeutic Drug Monitoring. 37 (1): 110–118. doi:10.1097/FTD.0000000000000113. PMC 5167556. PMID 24977379.
