This article may be too technical for most readers to understand. (February 2022) |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | completely absorbed at around 5 hours, steady state is reached by 60th hour |
| Protein binding | low (16%) |
| Metabolism | minimal |
| Elimination half-life | 7 to 12 hours |
| Excretion | mainly renal (unchanged), exposure is increased in renal impairment – on average by four-fold in subjects with severe renal impairment (CrCl <30 ml/min) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.023.355 |
| Chemical and physical data | |
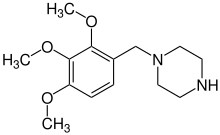
| Formula | C14H22N2O3 |
| Molar mass | 266.341 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Trimetazidine (IUPAC: 1-(2,3,4-trimethoxybenzyl)piperazine) is a drug sold under many brand names for angina pectoris (chest pain associated with impaired blood flow to the heart).[1] Trimetazidine is described as the first cytoprotective anti-ischemic agent developed and marketed by Laboratoires Servier (France). It is an anti-ischemic (antianginal) metabolic agent of the fatty acid oxidation inhibitor class, meaning that it improves the heart muscle's ability to use glucose as a fuel by inhibiting its use of fatty acid metabolism. It has become controversial for its use as a performance-enhancing drug, with several scandals involving its use erupting at successive Olympic games.
- ^ "Trimetazidine". Drugs.com.