| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trimethyl phosphite[1] | |||
| Other names
Trimethoxyphosphine
Trimethoxyphosphane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.065 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H9O3P | |||
| Molar mass | 124.08 | ||
| Appearance | colorless liquid | ||
| Odor | distinctive, pungent[2] | ||
| Density | 1.052 | ||
| Melting point | −78 °C (−108 °F; 195 K) | ||
| Boiling point | 111 °C (232 °F; 384 K) | ||
| reacts[2] | |||
| Vapor pressure | 24 mmHg (25°C)[2] | ||
| Hazards | |||
| Flash point | 28 °C; 82 °F; 301 K[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
REL (Recommended)
|
TWA 2 ppm (10 mg/m3)[2] | ||
IDLH (Immediate danger)
|
N.D.[2] | ||
| Related compounds | |||
Related compounds
|
Dimethyl methylphosphonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
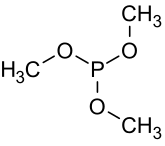
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 931. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0640". National Institute for Occupational Safety and Health (NIOSH).

