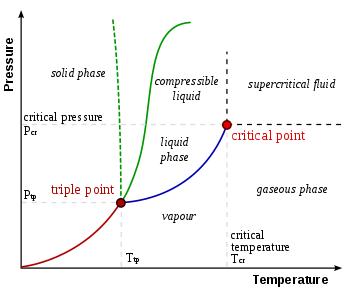
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.[1] It is that temperature and pressure at which the sublimation, fusion, and vaporisation curves meet. For example, the triple point of mercury occurs at a temperature of −38.8 °C (−37.8 °F) and a pressure of 0.165 mPa.
In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphs. Helium-4 is unusual in that it has no sublimation/deposition curve and therefore no triple points where its solid phase meets its gas phase. Instead, it has a vapor-liquid-superfluid point, a solid-liquid-superfluid point, a solid-solid-liquid point, and a solid-solid-superfluid point. None of these should be confused with the Lambda Point, which is not any kind of triple point.
The term "triple point" was coined in 1873 by James Thomson, brother of Lord Kelvin.[2] The triple points of several substances are used to define points in the ITS-90 international temperature scale, ranging from the triple point of hydrogen (13.8033 K) to the triple point of water (273.16 K, 0.01 °C, or 32.018 °F).
Before 2019, the triple point of water was used to define the kelvin, the base unit of thermodynamic temperature in the International System of Units (SI).[3] The kelvin was defined so that the triple point of water is exactly 273.16 K, but that changed with the 2019 revision of the SI, where the kelvin was redefined so that the Boltzmann constant is exactly 1.380649×10−23 J⋅K−1, and the triple point of water became an experimentally measured constant.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1994) "Triple point". doi:10.1351/goldbook.T06502.
- ^ James Thomson (1873) "A quantitative investigation of certain relations between the gaseous, the liquid, and the solid states of water-substance", Proceedings of the Royal Society, 22 : 27–36. From a footnote on page 28: "... the three curves would meet or cross each other in one point, which I have called the triple point".
- ^ Definition of the kelvin at BIPM.