| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cycloheptatrienylium[3] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1902352[1] | |||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C 7H+ 7[2] | |||
| Molar mass | 91.132 g·mol−1 | ||
| Structure | |||
| D7h | |||
| regular heptagon | |||
| Related compounds | |||
Other anions
|
Tropylium tetrafluoroborate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
The tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of [C7H7]+.[4] Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation can be stable, even with nucleophiles of moderate strength e.g., tropylium tetrafluoroborate and tropylium bromide (see below). Its bromide and chloride salts[5] can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively.[6]
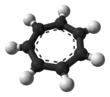
It is a regular heptagonal, planar, cyclic ion. It has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms. The structure shown is a composite of seven resonance contributors in which each carbon atom carries part of the positive charge.
- ^ a b c d e f g "tropylium | ChemSpider". www.chemspider.com. p. Names. Retrieved 30 December 2018.
tropylium
- ^ a b c d e f "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
Chemical Names: Tropylium; Cycloheptatrienylium; Cyc-C
7H+
7; Cyclohepta-2,4,6-trienylium - ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1127. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "molecule". doi:10.1351/goldbook.M04002
- ^ A mixture of [C7H7]+Cl− and [C7H7]+[PCl−
6] is produced by treatment of tropylidene with phosphorus pentachloride. - ^ Tropylium fluoborate Organic Syntheses, Coll. Vol. 5, p.1138 (1973); Vol. 43, p.101 (1963). link Archived 2012-08-29 at the Wayback Machine