| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hexacarbonyltungsten
| |||
| Other names
Tungsten carbonyl
Hexacarbonylwolfram | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.034.423 | ||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6O6W | |||
| Molar mass | 351.901 g/mol | ||
| Appearance | Colorless solid | ||
| Density | 2.65 g/cm3 | ||
| Melting point | 170 °C (338 °F; 443 K) (decomposes) | ||
| insoluble | |||
| Solubility | sparingly in THF | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable, CO source | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | External SDS | ||
| Related compounds | |||
Other cations
|
Chromium hexacarbonyl Molybdenum hexacarbonyl | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
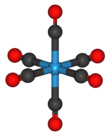
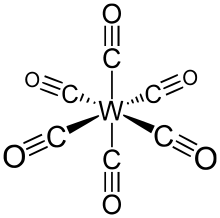
Tungsten hexacarbonyl (also called tungsten carbonyl) is an organometallic compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.[2]
Like its chromium and molybdenum analogs, this colorless compound is noteworthy as a volatile, air-stable derivative of tungsten in its zero oxidation state.
- ^ Even, J.; Yakushev, A.; Dullmann, C. E.; Haba, H.; Asai, M.; Sato, T. K.; Brand, H.; Di Nitto, A.; Eichler, R.; Fan, F. L.; Hartmann, W.; Huang, M.; Jager, E.; Kaji, D.; Kanaya, J.; Kaneya, Y.; Khuyagbaatar, J.; Kindler, B.; Kratz, J. V.; Krier, J.; Kudou, Y.; Kurz, N.; Lommel, B.; Miyashita, S.; Morimoto, K.; Morita, K.; Murakami, M.; Nagame, Y.; Nitsche, H.; et al. (2014). "Synthesis and detection of a seaborgium carbonyl complex". Science. 345 (6203): 1491–3. Bibcode:2014Sci...345.1491E. doi:10.1126/science.1255720. PMID 25237098. S2CID 206558746. (subscription required)
- ^ Kubas, G. J., Metal Dihydrogen and σ-Bond Complexes, Kluwer Academic/Plenum Publishers: New York, 2001