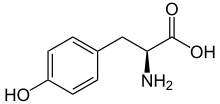
 Skeletal formula of L-tyrosine
| |||
 L-Tyrosine at physiological pH
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(S)-Tyrosine
| |||
| Other names
L-2-Amino-3-(4-hydroxyphenyl)propanoic acid
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.419 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C9H11NO3 | |||
| Molar mass | 181.191 g·mol−1 | ||
| Appearance | white solid | ||
| .0453 g/100 mL | |||
| -105.3·10−6 cm3/mol | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Supplementary data page | |||
| Tyrosine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
L-Tyrosine or tyrosine (symbol Tyr or Y)[2] or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese.[3][4] It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine.[5] It is encoded by the codons UAC and UAU in messenger RNA.
The one-letter symbol Y was assigned to tyrosine for being alphabetically nearest of those letters available. Note that T was assigned to the structurally simpler threonine, U was avoided for its similarity with V for valine, W was assigned to tryptophan, while X was reserved for undetermined or atypical amino acids.[6] The mnemonic tYrosine was also proposed.[7]
- ^ a b Frey MN, Koetzle TF, Lehmann MS, Hamilton WC (1973). "Precision neutron diffraction structure determination of protein and nucleic acid components. X. A comparison between the crystal and molecular structures of L-tyrosine and L-tyrosine hydrochloride". J. Chem. Phys. 58 (6): 2547–2556. Bibcode:1973JChPh..58.2547F. doi:10.1063/1.1679537.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ "Tyrosine". The Columbia Electronic Encyclopedia, 6th ed. Infoplease.com — Columbia University Press. 2007. Retrieved 2008-04-20.
- ^ Harper D (2001). "Tyrosine". Online Etymology Dictionary. Retrieved 2008-04-20.
- ^ "Amino Acids - Tyrosine". www.biology.arizona.edu. Retrieved 2018-01-31.
- ^ "IUPAC-IUB Commission on Biochemical Nomenclature A One-Letter Notation for Amino Acid Sequences". Journal of Biological Chemistry. 243 (13): 3557–3559. 10 July 1968. doi:10.1016/S0021-9258(19)34176-6.
- ^ Saffran M (April 1998). "Amino acid names and parlor games: from trivial names to a one-letter code, amino acid names have strained students' memories. Is a more rational nomenclature possible?". Biochemical Education. 26 (2): 116–118. doi:10.1016/S0307-4412(97)00167-2.