| Ubiquitin family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
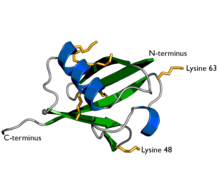 A diagram of ubiquitin. The seven lysine sidechains are shown in yellow/orange. | |||||||||
| Identifiers | |||||||||
| Symbol | ubiquitin | ||||||||
| Pfam | PF00240 | ||||||||
| InterPro | IPR000626 | ||||||||
| PROSITE | PDOC00271 | ||||||||
| SCOP2 | 1aar / SCOPe / SUPFAM | ||||||||
| |||||||||
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ubiquitously. It was discovered in 1975[1] by Gideon Goldstein and further characterized throughout the late 1970s and 1980s.[2] Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A.[3]
The addition of ubiquitin to a substrate protein is called ubiquitylation (or ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions.[4][5][6] Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, cysteine residues through a thioester bond, serine and threonine residues through an ester bond, or the amino group of the protein's N-terminus via a peptide bond.[7][8][9]
The protein modifications can be either a single ubiquitin protein (monoubiquitylation) or a chain of ubiquitin (polyubiquitylation). Secondary ubiquitin molecules are always linked to one of the seven lysine residues or the N-terminal methionine of the previous ubiquitin molecule. These 'linking' residues are represented by a "K" or "M" (the one-letter amino acid notation of lysine and methionine, respectively) and a number, referring to its position in the ubiquitin molecule as in K48, K29 or M1. The first ubiquitin molecule is covalently bound through its C-terminal carboxylate group to a particular lysine, cysteine, serine, threonine or N-terminus of the target protein. Polyubiquitylation occurs when the C-terminus of another ubiquitin is linked to one of the seven lysine residues or the first methionine on the previously added ubiquitin molecule, creating a chain. This process repeats several times, leading to the addition of several ubiquitins. Only polyubiquitylation on defined lysines, mostly on K48 and K29, is related to degradation by the proteasome (referred to as the "molecular kiss of death"), while other polyubiquitylations (e.g. on K63, K11, K6 and M1) and monoubiquitylations may regulate processes such as endocytic trafficking, inflammation, translation and DNA repair.[10]
The discovery that ubiquitin chains target proteins to the proteasome, which degrades and recycles proteins, was honored with the Nobel Prize in Chemistry in 2004.[8][11][12]
- ^ Cite error: The named reference
pmid1078892was invoked but never defined (see the help page). - ^ Wilkinson KD (October 2005). "The discovery of ubiquitin-dependent proteolysis". Proceedings of the National Academy of Sciences of the United States of America. 102 (43): 15280–2. Bibcode:2005PNAS..10215280W. doi:10.1073/pnas.0504842102. PMC 1266097. PMID 16230621.
- ^ Cite error: The named reference
pmid20418328was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid11917093was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid17218518was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid12860974was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid15571809was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
pmid22524316was invoked but never defined (see the help page). - ^ Cite error: The named reference
McDowell_2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid17609522was invoked but never defined (see the help page). - ^ "The Nobel Prize in Chemistry 2004". Nobelprize.org. Retrieved 2010-10-16.
- ^ "The Nobel Prize in Chemistry 2004: Popular Information". Nobelprize.org. Retrieved 2013-12-14.