| |
| Names | |
|---|---|
| IUPAC names
Uranium dioxide
Uranium(IV) oxide | |
| Other names
Urania
Uranous oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.014.273 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| UO2 | |
| Molar mass | 270.03 g/mol |
| Appearance | black powder |
| Density | 10.97 g/cm3 |
| Melting point | 2,865 °C (5,189 °F; 3,138 K) |
| insoluble | |
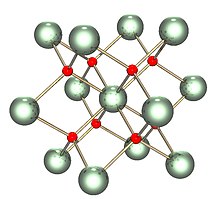
| Structure | |
| Fluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
a = 547.1 pm [1]
| |
| Tetrahedral (O2−); cubic (UIV) | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
78 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−1084 kJ·mol−1[2] |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H330, H373, H410 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | N/A |
| Safety data sheet (SDS) | ICSC 1251 |
| Related compounds | |
Other anions
|
Uranium(IV) sulfide Uranium(IV) selenide |
Other cations
|
Protactinium(IV) oxide Neptunium(IV) oxide |
| Triuranium octoxide Uranium trioxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Uranium dioxide or uranium(IV) oxide (UO2), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used as MOX fuel. Prior to 1960, it was used as yellow and black color in ceramic glazes and glass.
- ^ Leinders, Gregory; Cardinaels, Thomas; Binnemans, Koen; Verwerft, Marc (2015). "Accurate lattice parameter measurements of stoichiometric uranium dioxide". Journal of Nuclear Materials. 459: 135–42. Bibcode:2015JNuM..459..135L. doi:10.1016/j.jnucmat.2015.01.029. S2CID 97183844.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
