 | |
| Clinical data | |
|---|---|
| Trade names | Ingrezza |
| Other names | NBI-98854 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617023 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | >99% |
| Metabolism | Activation by hydrolysis, deactivation by CYP3A, CYP2D6 |
| Metabolites | [+]-α-Dihydrotetrabenazine (active metabolite) |
| Elimination half-life | 15–22 hrs |
| Excretion | 60% urine, 30% faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.236.234 |
| Chemical and physical data | |
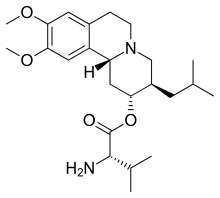
| Formula | C24H38N2O4 |
| Molar mass | 418.578 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Valbenazine, sold under the brand name Ingrezza, is a medication used to treat tardive dyskinesia.[1] It acts as a vesicular monoamine transporter 2 (VMAT2) inhibitor.[2]
- ^ a b "Ingrezza- valbenazine capsule; Ingrezza- valbenazine kit". DailyMed. 18 August 2023. Retrieved 17 November 2023.
- ^ O'Brien CF, Jimenez R, Hauser RA, Factor SA, Burke J, Mandri D, et al. (October 2015). "NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: A randomized, double-blind, placebo-controlled study". Movement Disorders. 30 (12): 1681–7. doi:10.1002/mds.26330. PMC 5049616. PMID 26346941.