| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Vanadium tetrachloride
Vanadium(IV) chloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.692 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| VCl4 | |||
| Molar mass | 192.75 g/mol | ||
| Appearance | bright red liquid, moisture sensitive | ||
| Odor | pungent | ||
| Density | 1.816 g/cm3, liquid | ||
| Melting point | −24.5 °C (−12.1 °F; 248.7 K) | ||
| Boiling point | 148 °C (298 °F; 421 K) | ||
| decomposes | |||
| Solubility | soluble in CH2Cl2 | ||
| Vapor pressure | 7.9 Pa | ||
| +1130.0·10−6 cm3/mol | |||
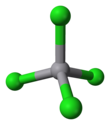
| Structure | |||
| tetrahedral | |||
| 0 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
toxic; oxidizer; hydrolyzes to release HCl | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
160 mg/kg (rat, oral) | ||
| Related compounds | |||
Other anions
|
vanadium tetrafluoride, vanadium disulfide, vanadium tetrabromide | ||
Other cations
|
titanium tetrachloride, chromium tetrachloride, niobium tetrachloride, tantalum tetrachloride | ||
Related compounds
|
vanadium trichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanadium tetrachloride is the inorganic compound with the formula VCl4. This reddish-brown liquid serves as a useful reagent for the preparation of other vanadium compounds.