 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɛməˈræfənɪb/ VEM-ə-RAF-ə-nib |
| Trade names | Zelboraf |
| Other names | PLX4032, RG7204, PLX4720, RO5185426 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.287.801 |
| Chemical and physical data | |
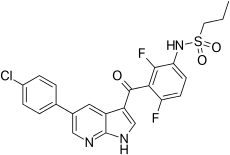
| Formula | C23H18ClF2N3O3S |
| Molar mass | 489.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
| vemurafenib | |
|---|---|
| Drug mechanism | |
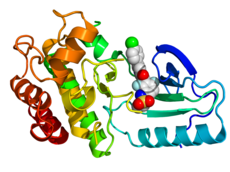 Crystallographic structure of B-Raf (rainbow colored, N-terminus = blue, C-terminus = red) complexed with vemurafenib (spheres, carbon = white, oxygen = red, nitrogen = blue, chlorine = green, fluorine = cyan, sulfur = yellow).[2] | |
| Therapeutic use | melanoma |
| Biological target | BRAF |
| Mechanism of action | protein kinase inhibitor |
| External links | |
| PDB ligand id | 032: PDBe, RCSB PDB |
| LIGPLOT | 3og7 |
Vemurafenib (INN), sold under the brand name Zelboraf, is a medication used for the treatment of late-stage melanoma.[2] It is an inhibitor of the B-Raf enzyme and was developed by Plexxikon.[2]
- ^ a b "Australian Product Information: Zelboraf® (vemurafenib)". Roche Products Pty Limited. 25 March 2020.
- ^ a b c PDB: 3OG7; Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. (September 2010). "Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma". Nature. 467 (7315): 596–599. Bibcode:2010Natur.467..596B. doi:10.1038/nature09454. PMC 2948082. PMID 20823850.