 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /vɛˈræpəmɪl/ ve-RAP-ə-mil |
| Trade names | Isoptin, Calan, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684030 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | Calcium channel blocker |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 35.1% |
| Metabolism | Liver |
| Onset of action | 1 to 2 hours (oral); 3 to 5 minutes (IV bolus)[6][7] |
| Elimination half-life | 2.8–7.4 hours[8] |
| Excretion | Kidney: 11% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.133 |
| Chemical and physical data | |
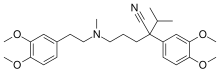
| Formula | C27H38N2O4 |
| Molar mass | 454.611 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Verapamil, sold under various trade names,[1] is a calcium channel blocker medication used for the treatment of high blood pressure, angina (chest pain from not enough blood flow to the heart), and supraventricular tachycardia.[9] It may also be used for the prevention of migraines and cluster headaches.[10][11] It is given by mouth or by injection into a vein.[9]
Common side effects include headache, low blood pressure, nausea, and constipation.[9] Other side effects include allergic reactions and muscle pains.[12] It is not recommended in people with a slow heart rate or heart failure.[12] It is believed to cause problems for the fetus if used during pregnancy.[2] It is in the non–dihydropyridine calcium channel blocker family of medications.[9]
Verapamil was approved for medical use in the United States in 1981.[9][13] It is on the World Health Organization's List of Essential Medicines.[14][15] Verapamil is available as a generic medication.[9] Long acting formulations exist.[12] In 2022, it was the 188th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[16][17]
- ^ a b "Verapamil". www.drugs.com. Archived from the original on 1 August 2017. Retrieved 14 December 2016.
- ^ a b "Verapamil Use During Pregnancy". Drugs.com. 18 November 2019. Archived from the original on 30 October 2024. Retrieved 26 March 2020.
- ^ "Securon SR - Summary of Product Characteristics (SmPC)". (emc). 17 May 2017. Archived from the original on 26 March 2020. Retrieved 26 March 2020.
- ^ Cite error: The named reference
Calan FDA labelwas invoked but never defined (see the help page). - ^ Human Medicines Evaluation Division (14 October 2020). "Active substance(s): verapamil". List of nationally authorised medicinal products. European Medicines Agency. Archived from the original (PDF) on 30 October 2024.
- ^ Cite error: The named reference
medicine-dosagewas invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid346345was invoked but never defined (see the help page). - ^ Schroeder JS, Frishman WH, Parker JD, et al. (2013). "Pharmacologic Options for Treatment of Ischemic Disease". Cardiovascular Therapeutics: A Companion to Braunwald's Heart Disease. Elsevier. pp. 83–130. doi:10.1016/b978-1-4557-0101-8.00007-2. ISBN 978-1-4557-0101-8.
The elimination half-life of standard verapamil tablets is usually 3 to 7 hours,...
- ^ a b c d e f "Verapamil Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ Tfelt-Hansen PC, Jensen RH (July 2012). "Management of cluster headache". CNS Drugs. 26 (7): 571–580. doi:10.2165/11632850-000000000-00000. PMID 22650381. S2CID 22522914.
- ^ Merison K, Jacobs H (November 2016). "Diagnosis and Treatment of Childhood Migraine". Current Treatment Options in Neurology. 18 (11): 48. doi:10.1007/s11940-016-0431-4. PMID 27704257. S2CID 28302667.
- ^ a b c World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. hdl:10665/44053. ISBN 9789241547659.
- ^ "Isoptin: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 30 October 2024. Retrieved 26 March 2020.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Verapamil Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.