 | |
| Clinical data | |
|---|---|
| Trade names | Verquvo |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Soluble guanylate cyclase activator |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.370 |
| Chemical and physical data | |
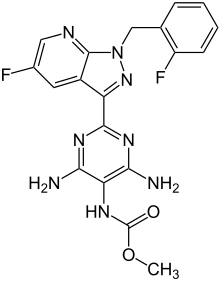
| Formula | C19H16F2N8O2 |
| Molar mass | 426.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vericiguat, sold under the brand name Verquvo, is a medication used to reduce the risk of cardiovascular death and hospitalization in certain patients with heart failure after a recent acute decompensation event.[3][4][8] It is taken by mouth.[3][4][8] Vericiguat is a soluble guanylate cyclase (sGC) stimulator.[3]
Common side effects include low blood pressure and low red cell count (anemia).[4][8]
It was approved for medical use in the United States in January 2021,[4][9] and for use in the European Union in July 2021.[8] The U.S. Food and Drug Administration considers it to be a first-in-class medication.[10]
- ^ a b "Verquvo". Therapeutic Goods Administration (TGA). 29 November 2021. Retrieved 28 December 2021.
- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Retrieved 13 May 2022.
- ^ a b c d e "Verquvo- vericiguat tablet, film coated". DailyMed. Retrieved 9 February 2021.
- ^ a b c d e "Drug Trials Snapshot: Verquvo". U.S. Food and Drug Administration (FDA). 8 February 2021. Retrieved 8 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Details for: Verquvo". Health Canada. 19 September 2023. Retrieved 3 March 2024.
- ^ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-06-23]". Health Canada. 23 June 2023. Retrieved 3 January 2024.
- ^ a b c d e Cite error: The named reference
Verquvo EPARwas invoked but never defined (see the help page). - ^ "Drug Approval Package: Verquvo". U.S. Food and Drug Administration (FDA). 17 February 2021. Retrieved 14 September 2021.
- ^ Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (PDF). U.S. Food and Drug Administration (FDA) (Report). 13 May 2022. Archived from the original on 6 December 2022. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.