 | |
| Clinical data | |
|---|---|
| Trade names | Brinavess |
| Other names | RSD1235 |
| Routes of administration | Intravenous,[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | low |
| Metabolism | CYP2D6, glucuronidation |
| Elimination half-life | 3–5.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.121.790 |
| Chemical and physical data | |
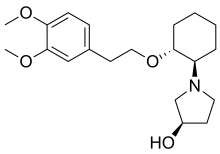
| Formula | C20H31NO4 |
| Molar mass | 349.471 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Vernakalant, sold under the brand name Brinavess, is a class III antiarrhythmic drug for the acute conversion of atrial fibrillation, a kind of irregular heartbeat, in form of an intravenous infusion. It has been approved for use in the European Union and the United Kingdom since 2010. The US Food and Drug Administration denied approval in 2008 and 2019.
- ^ "Brinavess: EPAR – Product information" (PDF). European Medicines Agency. 19 December 2019.
- ^ "Heart health". Health Canada. 9 May 2018. Retrieved 13 April 2024.