| |
| Names | |
|---|---|
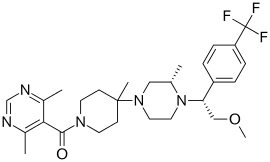
| IUPAC name
5-({4-[(3S)-4-{2-methoxy-1-[4-(trifluoromethyl)phenyl]ethyl}-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl}carbonyl)-4,6-dimethylpyrimidine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Vicriviroc |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H38F3N5O2 | |
| Molar mass | 533.629 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.