 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 56.6 ± 8.9% |
| Metabolism | hepatic |
| Elimination half-life | 2.54 ± 0.48 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.917 |
| Chemical and physical data | |
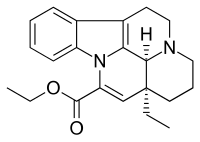
| Formula | C22H26N2O2 |
| Molar mass | 350.462 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Vinpocetine (ethyl apovincaminate) is a synthetic derivative of the vinca alkaloid vincamine, differing by the removal of a hydroxyl group and by being the ethyl rather than the methyl ester of the underlying carboxylic acid. Vincamine is extracted from either the seeds of Voacanga africana or the leaves of Vinca minor (lesser periwinkle).
- ^ Cite error: The named reference
FDA2019Pregwas invoked but never defined (see the help page). - ^ "Vinpocetine in Dietary Supplements". FDA. February 22, 2023. Retrieved June 9, 2023.