 | |
| Clinical data | |
|---|---|
| Trade names | Voquezna, others |
| AHFS/Drugs.com | Voquezna |
| MedlinePlus | a624006 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Potassium-competitive acid blocker |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 80% |
| Metabolism | Liver, by cytochrome P450 (3A4, 2B6, 2C19, 2D6) |
| Elimination half-life | 7.7 h |
| Duration of action | > 24 h |
| Excretion | Kidney |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
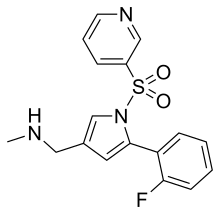
| Formula | C17H16FN3O2S |
| Molar mass | 345.39 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vonoprazan, sold under the brand name Voquezna among others, is a first-in-class potassium-competitive acid blocker medication.[2][1] Vonoprazan is used in form of the fumarate for the treatment of gastroduodenal ulcer (including some drug-induced peptic ulcers) and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori.[2]
It was approved in Japan in February 2015,[3][4] and in Russia in April 2021.[5] Vonoprazan was approved for medical use in the United States in November 2023.[1][6] Co-packaged combinations of vonoprazan with amoxicillin and vonoprazan with amoxicillin and clarithromycin are available.[7][8]
- ^ a b c "Voquezna- vonoprazan fumarate tablet". DailyMed. 8 November 2023. Retrieved 20 November 2023.
- ^ a b Echizen H (April 2016). "The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations". Clinical Pharmacokinetics. 55 (4): 409–418. doi:10.1007/s40262-015-0326-7. PMID 26369775. S2CID 5984975.
- ^ Garnock-Jones KP (2015). "Vonoprazan: first global approval". Drugs. 75 (4): 439–43. doi:10.1007/s40265-015-0368-z. PMID 25744862. S2CID 43293048.
- ^ "Takecab Now Available for the Treatment of Acid-related Diseases in Japan". Takeda Pharmaceuticals (Press release). 26 February 2015. Archived from the original on 26 October 2023. Retrieved 20 November 2023.
- ^ "Russian State Register of Medicines. Vocinti (vonoprazan) Film-Coated Tablets. Full Prescribing Information". grls.rosminzdrav.ru (in Russian). Takeda Pharmaceutical Company Limited. Archived from the original on 18 February 2023. Retrieved 18 February 2023.
- ^ "Phathom Pharmaceuticals Announces Commercial Availability of Voquezna (vonoprazan) Tablets, a Powerful First-In-Class PCAB for the Treatment of Erosive GERD and Relief of Associated Heartburn". GlobeNewswire (Press release). Phathom Pharmaceuticals. 28 November 2023. Retrieved 26 January 2024.
- ^ "Voquezna Dual Pak- vonoprazan fumarate and amoxicillin kit; Voquezna Triple Pak- vonoprazan fumarate, amoxicillin and clarithromycin kit". DailyMed. 21 June 2022. Archived from the original on 3 July 2022. Retrieved 3 July 2022.
- ^ "Phathom Pharmaceuticals Announces FDA Approval of Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) for the Treatment of H. pylori Infection in Adults" (Press release). Phathom Pharmaceuticals. 3 May 2022. Archived from the original on 10 May 2022. Retrieved 9 May 2022 – via GlobeNewswire.