| White adipose tissue | |
|---|---|
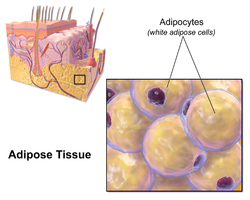 Illustration depicting white fat cells. | |
| Details | |
| Identifiers | |
| Latin | textus adiposus albus |
| MeSH | D052436 |
| TH | H2.00.03.4.00002 |
| FMA | 20117 |
| Anatomical terminology | |
White adipose tissue or white fat is one of the two types of adipose tissue found in mammals. The other kind is brown adipose tissue. White adipose tissue is composed of monolocular adipocytes.
In humans, the healthy amount of white adipose tissue varies with age, but composes between 6–25% of body weight in adult men and 14–35% in adult women.[1][additional citation(s) needed]
Its cells contain a single large fat droplet, which forces the nucleus to be squeezed into a thin rim at the periphery. They have receptors for insulin, sex hormones, norepinephrine, and glucocorticoids.
White adipose tissue is used for energy storage. Upon release of insulin from the pancreas, white adipose cells' insulin receptors cause a dephosphorylation cascade that leads to the inactivation of hormone-sensitive lipase. It was previously thought that upon release of glucagon from the pancreas, glucagon receptors cause a phosphorylation cascade that activates hormone-sensitive lipase, causing the breakdown of the stored fat to fatty acids, which are exported into the blood and bound to albumin, and glycerol, which is exported into the blood freely. There is actually no evidence at present that glucagon has any effect on lipolysis in white adipose tissue.[2] Glucagon is now thought to act exclusively on the liver to trigger glycogenolysis and gluconeogenesis.[3] The trigger for this process in white adipose tissue is instead now thought to be adrenocorticotropic hormone,[4][5] adrenaline[6] and noradrenaline[citation needed]. Fatty acids are taken up by muscle and cardiac tissue as a fuel source, and glycerol is taken up by the liver for gluconeogenesis.
White adipose tissue also acts as a thermal insulator, helping to maintain body temperature.
The hormone leptin is primarily manufactured in the adipocytes of white adipose tissue[7] which also produces another hormone, asprosin.
- ^ AACE/ACE Obesity Task Force (1998). "AACE/ACE position statement on the prevention, diagnosis, and treatment of obesity". Endocr Pract. 4 (5).
- ^ Gravholt CH, Møller N, Jensen MD, Christiansen JS, Schmitz O (May 2001). "Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis". The Journal of Clinical Endocrinology and Metabolism. 86 (5): 2085–9. doi:10.1210/jcem.86.5.7460. PMID 11344211.
- ^ Lawrence AM (1969). "Glucagon". Annual Review of Medicine. 20: 207–22. doi:10.1146/annurev.me.20.020169.001231. PMID 4893399.
- ^ Spirovski MZ, Kovacev VP, Spasovska M, Chernick SS (February 1975). "Effect of ACTH on lipolysis in adipose tissue of normal and adrenalectomized rats in vivo". The American Journal of Physiology. 228 (2): 382–5. doi:10.1152/ajplegacy.1975.228.2.382. PMID 164126.
- ^ Kiwaki K, Levine JA (November 2003). "Differential effects of adrenocorticotropic hormone on human and mouse adipose tissue". Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 173 (8): 675–8. doi:10.1007/s00360-003-0377-1. PMID 12925881. S2CID 12459319.
- ^ Stallknecht B, Simonsen L, Bülow J, Vinten J, Galbo H (December 1995). "Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue". The American Journal of Physiology. 269 (6 Pt 1): E1059-66. doi:10.1152/ajpendo.1995.269.6.E1059. PMID 8572197.
- ^ Zhou Y, Rui L (June 2013). "Leptin signaling and leptin resistance". Frontiers of Medicine. 7 (2): 207–22. doi:10.1007/s11684-013-0263-5. PMC 4069066. PMID 23580174.