| |

| |
| Names | |
|---|---|
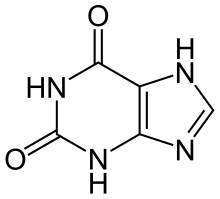
| Preferred IUPAC name
3,7-Dihydro-1H-purine-2,6-dione | |
| Other names
1H-Purine-2,6-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.653 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H4N4O2 | |
| Molar mass | 152.11 g/mol |
| Appearance | White solid |
| Melting point | decomposes |
| 1 g/ 14.5 L @ 16 °C 1 g/1.4 L @ 100 °C | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xanthine (/ˈzænθiːn/ or /ˈzænθaɪn/, from Ancient Greek ξανθός xanthós 'yellow' for its yellowish-white appearance; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms.[2] Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine.[3][4]
Xanthine is a product on the pathway of purine degradation.[2]
- It is created from guanine by guanine deaminase.
- It is created from hypoxanthine by xanthine oxidoreductase.
- It is also created from xanthosine by purine nucleoside phosphorylase.
Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme.[2]
- ^ Merck Index, 11th Edition, 9968.
- ^ a b c "Xanthine, CID 1188". PubChem, National Library of Medicine, US National Institutes of Health. 2019. Retrieved 28 September 2019.
- ^ Spiller, Gene A. (1998). Caffeine. Boca Raton: CRC Press. ISBN 0-8493-2647-8.
- ^ Katzung, Bertram G. (1995). Basic & Clinical Pharmacology. East Norwalk, Connecticut: Paramount Publishing. pp. 310, 311. ISBN 0-8385-0619-4.
