 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌzaɪloʊˌmɛtəˈzoʊliːn/ ZY-lo-MET-ə-ZOH-leen |
| Trade names | Otrivin, Otrivine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608026 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Moderate[1] |
| Routes of administration | intranasal (spray or drops) |
| Drug class | α1 and α2 Adrenergic receptor agonist, Decongestant |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 2–3 hours [citation needed] |
| Excretion | Urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.629 |
| Chemical and physical data | |
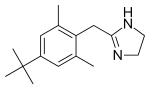
| Formula | C16H24N2 |
| Molar mass | 244.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Xylometazoline, also spelled xylomethazoline, is a drug used to reduce symptoms of nasal congestion, allergic rhinitis, and sinusitis.[2] Use is not recommended for more than seven days.[3] Use is also not recommended in those less than three months of age and some say not less than 6 years of age.[3][4] It is used directly in the nose as a spray or drops.[3]
Side effects include trouble sleeping, irritation of the nose, nausea, nosebleed (3%), period pain (10%) and headache (3%).[5][2][3] Long term use (> 10 days) is not recommended due to a rhinitis medicamentosa when stopped.[5][6] Use is not recommended during pregnancy.[2] Xylometazoline is in the decongestant and alpha-adrenergic agonist families of medication.[6][7]
One study classified it with selectivity ratios in alpha 2 adrenergic receptors of 151 for a2A vs a2B, 4.5 a2A vs a2C, and 33.9 a2B vs a2C. Making it a highly selective a2A agonist.[8]
Xylometazoline was patented in 1956 and came into medical use in 1959.[9][10] It is on the World Health Organization's List of Essential Medicines.[4][11] Xylometazoline is available as a generic medication.[3]
- ^ Fulga A, Zenovia A, Ene DC, Stan C, Firescu D, Fulga I (18 November 2021). "Fulga, Ana, Andrei Zenovia, Doriana Cristea Ene, Constantin Stan, Dorel Firescu, and Iuliu Fulga. 2021. "Addiction to Nasal Decongestants Based on Α-Adrenoceptor Agonists Case Series and Literature Review: Array". EuroEconomica 40 (2)". Euroeconomica. 40 (2).
- ^ a b c "Otrivine Adult Measured Dose Sinusitis Spray - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. 13 April 2016. Archived from the original on 29 December 2016. Retrieved 28 December 2016.
- ^ a b c d e British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 786. ISBN 978-0-85711-156-2.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b Eccles R, Martensson K, Chen SC (April 2010). "Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold". Current Medical Research and Opinion. 26 (4): 889–899. doi:10.1185/03007991003648015. PMID 20151787. S2CID 34728458.
- ^ a b Graf P (1997). "Rhinitis medicamentosa: aspects of pathophysiology and treatment". Allergy. 52 (40 Suppl): 28–34. doi:10.1111/j.1398-9995.1997.tb04881.x. PMID 9353558. S2CID 72326981.
- ^ "Xylometazoline nasal medical facts from Drugs.com". www.drugs.com. Archived from the original on 29 December 2016. Retrieved 28 December 2016.
- ^ Proudman RG, Akinaga J, Baker JG (October 2022). "The signaling and selectivity of α-adrenoceptor agonists for the human α2A, α2B and α2C-adrenoceptors and comparison with human α1 and β-adrenoceptors". Pharmacology Research & Perspectives. 10 (5): e01003. doi:10.1002/prp2.1003. PMC 9471048. PMID 36101495.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 552. ISBN 978-3-527-60749-5. Archived from the original on 29 December 2016.
- ^ US patent 2868802A, Hüni, Albrecht, "2-(γ-TERT-BUTYL-O,O'-DIMETHYL-PHENYL-METHYL)-IMIDAZOLINE AND SALTS", issued 1959-01-13, assigned to Ciba Pharmaceutical Products Inc., Summit, N. J.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.