
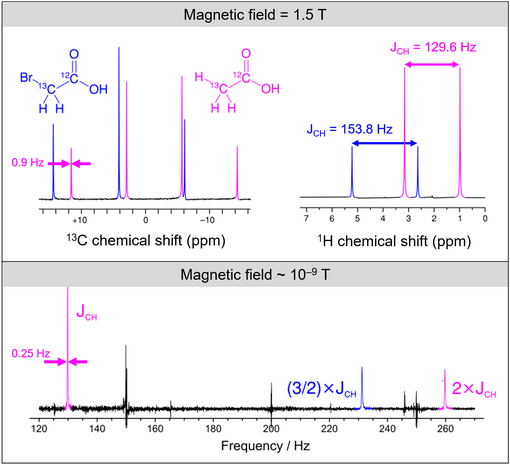
Zero- to ultralow-field (ZULF) NMR is the acquisition of nuclear magnetic resonance (NMR) spectra of chemicals with magnetically active nuclei (spins 1/2 and greater) in an environment carefully screened from magnetic fields (including from the Earth's field). ZULF NMR experiments typically involve the use of passive or active shielding to attenuate Earth’s magnetic field. This is in contrast to the majority of NMR experiments which are performed in high magnetic fields provided by superconducting magnets. In ZULF experiments the sample is moved through a low field magnet into the "zero field" region where the dominant interactions are nuclear spin-spin couplings, and the coupling between spins and the external magnetic field is a perturbation to this. There are a number of advantages to operating in this regime: magnetic-susceptibility-induced line broadening is attenuated which reduces inhomogeneous broadening of the spectral lines for samples in heterogeneous environments. Another advantage is that the low frequency signals readily pass through conductive materials such as metals due to the increased skin depth; this is not the case for high-field NMR for which the sample containers are usually made of glass, quartz or ceramic. [2] High-field NMR employs inductive detectors to pick up the radiofrequency signals, but this would be inefficient in ZULF NMR experiments since the signal frequencies are typically much lower (on the order of hertz to kilohertz). The development of highly sensitive magnetic sensors in the early 2000s including SQUIDs, magnetoresistive sensors, and SERF atomic magnetometers made it possible to detect NMR signals directly in the ZULF regime. Previous ZULF NMR experiments relied on indirect detection where the sample had to be shuttled from the shielded ZULF environment into a high magnetic field for detection with a conventional inductive pick-up coil. One successful implementation was using atomic magnetometers at zero magnetic field working with rubidium vapor cells to detect zero-field NMR.[3][4]
Without a large magnetic field to induce nuclear spin polarization, the nuclear spins must be polarized externally using hyperpolarization techniques. This can be as simple as polarizing the spins in a magnetic field followed by shuttling to the ZULF region for signal acquisition, and alternative chemistry-based hyperpolarization techniques can also be used.
It is sometimes but inaccurately referred to as nuclear quadrupole resonance (NQR).[5]
- ^ Burueva, D.; Eills, J.; Blanchard, J.W.; Garcon, A.; Picazo Frutos, R.; Kovtunov, K.V.; Koptyug, I.; Budker, D. (June 8, 2020). "Chemical Reaction Monitoring using Zero-Field Nuclear Magnetic Resonance Enables Study of Heterogeneous Samples in Metal Containers". Angew. Chem. Int. Ed. 59 (39): 17026–17032. doi:10.1002/anie.202006266. PMC 7540358. PMID 32510813.
- ^ Put, Piotr; Pustelny, Szymon; Budker, Dmitry; Druga, Emanuel; Sjolander, Tobias F.; Pines, Alexander; Barskiy, Danila A. (2021). "Zero- to Ultralow-Field NMR Spectroscopy of Small Biomolecules". Analytical Chemistry. 93 (6): 3226–3232. doi:10.1021/acs.analchem.0c04738.
- ^ Sheng, D.; Li, S.; Dural, N.; Romalis, M. (18 April 2013). "Subfemtotesla Scalar Atomic Magnetometry Using Multipass Cells". Physical Review Letters. 110 (16): 160802. arXiv:1208.1099. Bibcode:2013PhRvL.110p0802S. doi:10.1103/PhysRevLett.110.160802. PMID 23679590. S2CID 7559023.
- ^ Commissariat, Tushna (April 24, 2013). "Atomic magnetometer is most sensitive yet". Physics World.
- ^ U.S. patent 6,919,838