| |

| |
| Names | |
|---|---|
| Other names
Zinc diperchlorate, zinc(II) perchlorate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.733 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
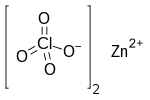
| Cl 2O 8Zn | |
| Molar mass | 261.826 |
| Appearance | colorless solid |
| Density | 2.252 g/cm3 |
| Melting point | 106 °C (223 °F; 379 K) |
| Boiling point | 210 °C (410 °F; 483 K) |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zinc perchlorate is the inorganic compound with the chemical formula Zn(ClO4)2 which forms the hexahydrate.[1][2]
- ^ Kumar, Raj; Thilagavathi, Ramasamy; Gulhane, Rajesh; Chakraborti, Asit K. (2 May 2006). "Zinc(II) perchlorate as a new and highly efficient catalyst for formation of aldehyde 1,1-diacetate at room temperature and under solvent-free conditions". Journal of Molecular Catalysis A: Chemical. 250 (1): 226–231. doi:10.1016/j.molcata.2006.01.063. ISSN 1381-1169. Retrieved 14 March 2023.
- ^ Advances in Inorganic Chemistry. Academic Press. 5 December 1984. p. 283. ISBN 978-0-08-057877-4. Retrieved 14 March 2023.