
| |
| |

| |
| Names | |
|---|---|
| IUPAC names
Zirconium tetrachloride
Zirconium(IV) chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.041 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| ZrCl4 | |
| Molar mass | 233.04 g/mol |
| Appearance | white crystals |
| Density | 2.80 g/cm3 |
| Melting point | 437 °C (819 °F; 710 K) (triple point) |
| Boiling point | 331 °C (628 °F; 604 K) (sublimes) |
| hydrolysis | |
| Solubility | concentrated HCl (with reaction) |
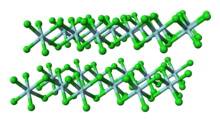
| Structure | |
| Monoclinic, mP10 | |
| P12/c1, No. 13 | |
| Thermochemistry | |
Heat capacity (C)
|
125.38 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
181.41 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−980.52 kJ/mol |
| Hazards | |
| GHS labelling:[2] | |
  
| |
| Danger | |
| H290, H302, H312, H314, H317, H332, H334 | |
| P234, P260, P261, P264, P270, P271, P272, P280, P285, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P304+P341, P305+P351+P338, P310, P312, P321, P322, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1488-1500 mg/kg (oral, rat) 655 mg/kg (mouse, oral)[1] |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions
|
Zirconium(IV) fluoride Zirconium(IV) bromide Zirconium(IV) iodide |
Other cations
|
Titanium tetrachloride Hafnium tetrachloride |
Related compounds
|
Zirconium(II) chloride, Zirconium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zirconium(IV) chloride, also known as zirconium tetrachloride, (ZrCl4) is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
- ^ "Zirconium compounds (as Zr)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ GHS: PubChem
- ^ "New Environment Inc. - NFPA Chemicals". newenv.com. Retrieved 26 April 2017.
