| Zona glomerulosa | |
|---|---|
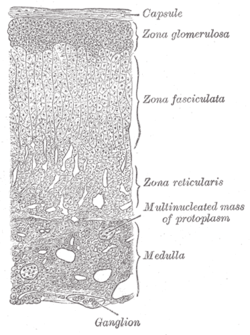 Layers of cortex. | |
| Details | |
| Identifiers | |
| Latin | zona glomerulosa |
| MeSH | D015384 |
| FMA | 69225 |
| Anatomical terminology | |
The zona glomerulosa (sometimes, glomerular zone) of the adrenal gland is the most superficial layer of the adrenal cortex, lying directly beneath the renal capsule. Its cells are ovoid and arranged in clusters or arches (glomus is Latin for "ball").[citation needed]

In response to increased potassium levels, renin or decreased blood flow to the kidneys, cells of the zona glomerulosa produce and secrete the mineralocorticoid aldosterone into the blood as part of the renin–angiotensin system.[1] Although sustained production of aldosterone requires persistent calcium entry through low-voltage activated Ca2+ channels, isolated zona glomerulosa cells are considered nonexcitable, with recorded membrane voltages that are too hyperpolarized to permit Ca2+ channels entry.[2] However, mouse zona glomerulosa cells within adrenal slices spontaneously generate membrane potential oscillations of low periodicity; this innate electrical excitability of these cells provides a platform for the production of a recurrent Ca2+ channels signal that can be controlled by angiotensin II and extracellular potassium, the 2 major regulators of aldosterone production.[2] Aldosterone regulates the body's concentration of electrolytes, primarily sodium and potassium, by acting on the distal convoluted tubule of kidney nephrons to: increase sodium reabsorption, increase potassium excretion, increase water reabsorption through osmosis.[1]
The enzyme aldosterone synthase (also known as CYP11B2) acts in this location[3][4] The expression of neuron-specific proteins in the zona glomerulosa cells of human adrenocortical tissues has been predicted and reported by several authors[5][6][7] and it was suggested that the expression of proteins like the neuronal cell adhesion molecule (NCAM) in the cells of the zona glomerulosa reflects the regenerative feature of these cells, which would lose NCAM immunoreactivity after moving to the zona fasciculata.[5][8] However, together with other data on neuroendocrine properties of zona glomerulosa cells, NCAM expression may reflect a neuroendocrine differentiation of these cells.[5] Voltage-dependent calcium channels have been detected in the zona glomerulosa of the human adrenal, which suggests that calcium-channel blockers may directly influence the adrenocortical biosynthesis of aldosterone in vivo.[9]
- ^ a b Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14
- ^ a b Hu, Changlong; Rusin, Craig G.; Tan, Zhiyong; Guagliardo, Nick A.; Barrett, Paula Q. (2012). "Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators". Journal of Clinical Investigation. 122 (6): 2046–53. doi:10.1172/JCI61996. PMC 3966877. PMID 22546854.
- ^ Curnow, K. M.; Tusie-Luna, M.-T.; Pascoe, L.; Natarajan, R.; Gu, J.-L.; Nadler, J. L.; White, P. C. (1991). "The Product of the CYP11B2 Gene is Required for Aldosterone Biosynthesis in the Human Adrenal Cortex". Molecular Endocrinology. 5 (10): 1513–22. doi:10.1210/mend-5-10-1513. PMID 1775135.
- ^ Zhou, M.Y.; Gomez-Sanchez, C.E. (1993). "Cloning and Expression of a Rat Cytochrome P-450 11β-Hydroxylase/Aldosterone Synthase (CYP11B2) cDNA Variant". Biochemical and Biophysical Research Communications. 194 (1): 112–7. doi:10.1006/bbrc.1993.1792. PMID 8333830.
- ^ a b c Ehrhart-Bornstein, M.; Hilbers, U. (1998). "Neuroendocrine Properties of Adrenocortical Cells" (PDF). Hormone and Metabolic Research. 30 (6/07): 436–439. doi:10.1055/s-2007-978911. PMID 9694576.
- ^ Lefebvre, H.; Cartier, D; Duparc, C; Lihrmann, I; Contesse, V; Delarue, C; Godin, M; Fischmeister, R; Vaudry, H; Kuhn, JM (2002). "Characterization of Serotonin4 Receptors in Adrenocortical Aldosterone-Producing Adenomas: In Vivo and in Vitro Studies". Journal of Clinical Endocrinology & Metabolism. 87 (3): 1211–6. doi:10.1210/jcem.87.3.8327. PMID 11889190.
- ^ Ye, P.; Mariniello, B.; Mantero, F.; Shibata, H.; Rainey, W. E (2007). "G-protein-coupled receptors in aldosterone-producing adenomas: A potential cause of hyperaldosteronism". Journal of Endocrinology. 195 (1): 39–48. doi:10.1677/JOE-07-0037. PMID 17911395.
- ^ Haidan, A.; Bornstein, SR; Glasow, A; Uhlmann, K; Lübke, C; Ehrhart-Bornstein, M (1998). "Basal Steroidogenic Activity of Adrenocortical Cells is Increased 10-Fold by Coculture with Chromaffin Cells". Endocrinology. 139 (2): 772–80. doi:10.1210/endo.139.2.5740. PMID 9449652.
- ^ Saulo J.A. Felizola; Takashi Maekawa; Yasuhiro Nakamura; Fumitoshi Satoh; Yoshikiyo Ono; Kumi Kikuchi; Shizuka Aritomi; Keiichi Ikeda; Michihiro Yoshimura; Katsuyoshi Tojo; Hironobu Sasano. (2014). "Voltage-gated calcium channels in the human adrenal and primary aldosteronism". J Steroid Biochem Mol Biol. 144 (part B): 410–416. doi:10.1016/j.jsbmb.2014.08.012. PMID 25151951.